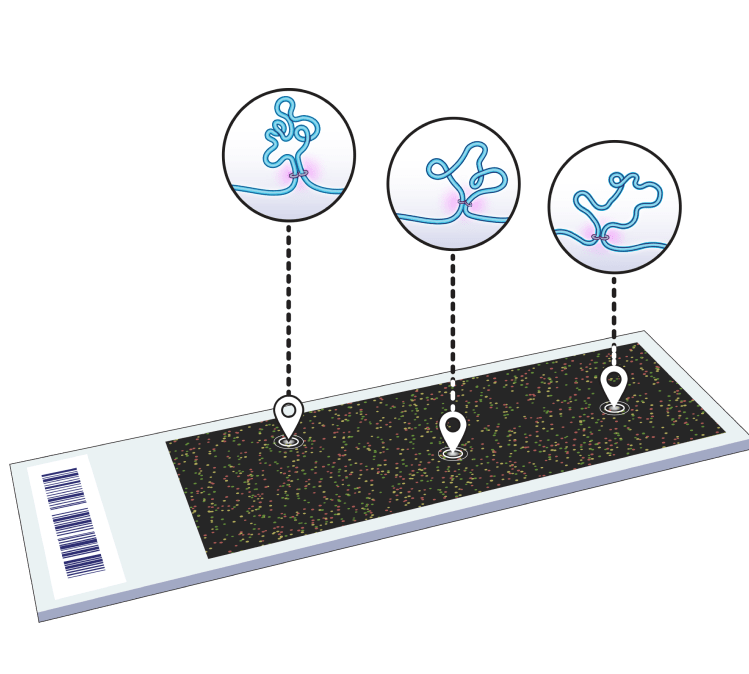
Enabling 3D genomics for precision medicine at scale
The EpiSwitch platform is uniquely capable of reproducibly translating 3D genome regulation for clinical application. This platform opens the doors to a portfolio of clinical smart tests based on EpiSwitch® technology aiming to help people face the most challenging health decisions with confidence and tackle the rising costs of healthcare.